Biochar enthusiasts know of the Amazonians, who used biochar to create an agricultural civilization in an inhospitable rainforest environment. But we are not so aware of biochar history in Europe and America in 19th century. It began with Justus Liebig, the “father of organic chemistry” who wrote that charcoal “surpasses all other substances in the power which it possesses of condensing ammonia within its pores… it absorbs 90 times its volume of ammoniacal gas, which may be again separated by simply moistening it with water.” (Agricultural Chemistry, p 35.) This simple statement launched a wave of practical experimentation using charcoal for agriculture and waste management that lasted for nearly a century. The evidence is found in hundreds of reports published in science and agriculture journals. Some of these early proponents of biochar developed visionary proposals for reforming agriculture and civilization. They articulated their proposals with the same urgency and moral sense that we use today when discussing issues like climate change, food security and energy security. In the 19th century, the issues that biochar could help solve were related to health, disease, poverty, and above all, the recycling of human sewage to replenish the soil.
Justus Liebig and the Overthrow of the Humus theory
The science of agricultural charcoal in the West began with the German chemist, Justus von Liebig. Liebig questioned the prevailing theory that humus or black soil was essential for plant growth because it imparted a vital life force to plants. Believers in humus thought that the black soil contained an organic life force or “vitalism” that could not be derived from dead, inorganic chemicals. This theory was based on the well-known fact that “virgin” soil from recently cleared forests was black and fertile.

 Figure 1. The young Justus Liebig.
Figure 1. The young Justus Liebig.
Liebig reasoned that this must be wrong. Humus was clearly the product of plant decay, so how could the first plants on earth have ever become established if humus was necessary? Supporting his theory, Liebig found that plants could be grown in charcoal – a “dead” chemical matter with no vital force. Some chemists proposed that plants needed humus as a source of carbon, but Liebig showed that humus was too insoluble to provide the needed carbon. Charcoal was even more recalcitrant than humus. He concluded that a plant that grows in charcoal must get its carbon from carbon dioxide in the air.
His scientific colleagues soon conceded that Liebig had “overthrown” the humus theory in favor of the chemical theory of agriculture. Through Liebig’s experimental work, he also established the famous “law of the minimum,” that states that plant growth is constrained by the least available nutrient in the soil. These discoveries spurred a growing fertilizer industry that mined and shipped huge amounts of guano, bonemeal, lime and other fertilizers from all parts of the world to fertilize the fields of Europe and eliminate the need for crop rotations and fallow periods to replenish the soil. Eventually, chemical processes were developed to manufacture fertilizers such as super phosphate and ammonia. Liebig’s chemical theory of agriculture was as key to the industrial revolution as Watt’s steam engine. Liebig certainly meant it to be. He said, “Perfect agriculture is the true foundation of all trade and industry.” (Agricultural Chemistry, p 3.)
But the success of the chemical fertilizer industry was not universally approved. Liebig himself had great misgivings about importation of fertilizers and the consequences for food security, “in the event of war with America when supplies of guano would cease.” (Brock, p 257.) Imports were especially troublesome given the growing issue of town sewage disposal. Increasingly, London and other cities were flushing waste to the sea that had formerly been used to fertilize crops. Liebig’s voice was one of many that decried the wastage of “night soil” (a term for human excreta). Liebig had read accounts of Chinese agriculture that led him to declare: “But how infinitely inferior is the agriculture of Europe to that of China! The Chinese are the most admirable gardeners and trainers of plants…the agriculture of their country is the most perfect in the world.” Perfect because the Chinese understood the importance of the “most important of all manures,” human excrement. Liebig said, “Indeed so much value is attached to the influence of human excrements by these people, that laws of the state forbid that any of them should be thrown away, and reservoirs are placed in every house, in which they are collected with the greatest care.” (Agricultural Chemistry, pp 65-66.)
Liebig would periodically try to influence policymakers who governed the development of the sewerage and waste treatment systems in the great cities of Europe, but ultimately to little effect.
The Passion for Charcoal
Liebig was a devoted experimentalist and he soon realized that humus did have a very important function in soil – it could absorb and hold chemical fertilizers that would otherwise leach away before plant roots could take them up. He compared the absorptivity of humus to that of charcoal and its ability, “surpassing all others” to absorb ammonia.
 Figure 2. Advertisement for poudrette made from human night soil and other ingredients manufactured by the Lodi Manufacturing Company in the United States in the 19th Century.
Figure 2. Advertisement for poudrette made from human night soil and other ingredients manufactured by the Lodi Manufacturing Company in the United States in the 19th Century.
Liebig cited a series of hothouse experiments (Appendix to Part I, Agricultural Chemistry, pp 84-86) that a colleague had conducted using mixtures of charcoal in potted plants that gave fantastic results: “An addition of charcoal, for example, to vegetable mould, appeared to answer excellently for the Gesneria and Gloxinia and also for the tropical Aroideae with tuberous roots. The two first soon excited the attention of connoisseurs, by the great beauty of all their parts and their general appearance.”
The hothouse experiments also revealed that charcoal could have dramatic effects on the health of plants: “Pure charcoal acts excellently as a means of curing unhealthy plants. A Dorianthes excelsa, for example, which had been drooping for three years, was rendered completely healthy in a very short time by this means. An orange tree, which had the very common disease in which the leaves become yellow, acquired within four weeks its healthy green color, when the upper surface of the earth was removed from the pot in which it was contained, and a ring of charcoal of an inch in thickness strewed in its place around the periphery of the pot. The same was the case with the Gardenia.”
Liebig did not have much to say about these hothouse results with charcoal – he cited them to prove his point about the superfluity of humus, but popular journals for horticulturalists and farmers soon took notice of these experiments and published descriptions, articles and testimonials on the beneficial effects of charcoal in soil. Many of the writers also observed that crops grown on old charcoal manufacturing sites, or on the kitchen middens of Indian villages were especially abundant. Here are several quotes of interest from this abundant literature:
“My attention was first drawn to the influence of charcoal, by the wonderful experiments of Baron Von Liebig, in the propagation of plants, and the facility with which cuttings were rooted in this substance. Its use became very general in Europe by amateurs and cultivators of plants… As a medium for storing up the volatile portions of manure and compost heaps…”(The Journal of Agriculture, 1851)
“For two years past I have used some fifty loads each season of refuse charcoal, and being fully convinced that it pays, I wish to recommend it to my brother farmers. I have tried it on grass, corn and potatoes—have tried it alone and in the compost heap, and in all situations it has proved faithful to its trust. As a top dressing for grass, it gives a green color and luxuriant growth. Applied to half an acre of early potatoes the last summer, the yield was 75 bushels of as fine healthy potatoes as could be desired, that sold readily for one dollar per bushel, and yielded the best profit of anything raised on the farm.” (The New Jersey Farmer, 1856)
“In the midst of the disastrous drouth of last summer, while crossing a field in Moriah, occupied by Mr. Richmond, in pursuit of some Durham cattle I wished to examine, I observed a lot with its surface deeply and singularly blackened. Upon inspection I found it thickly strewn with pulverized charcoal. The field presented a rich verdure, strongly contrasting with the parched and blighted aspect of the adjacent country.” (New York State Agricultural Society, 1853)
“Poudrette (night-soil deodorized with charcoal dust) is one of the best manures for the rose… Charcoal dust is an excellent surface dressing; it imbibes and retains moisture, keeps the plant healthy and intensifies the color of red varieties.” (The Garden Diary of Martha Turnbull)
“A dead rat, nicely buried in a cigar box so as to be surrounded at all points by an inch of charcoal powder, decays to bone and fur without manifesting any odour of putrefaction, so that it might stand on a parlour table and not reveal its contents to the most sensitive nostrils.” (The Garden, 1873)
Liebig’s Warnings
It is interesting to note that while industry quickly found the profits to be made in mining and manufacturing chemical fertilizers, it never grasped the need for replenishing soil carbon, either in the form of humus or charcoal. Despite the interest expressed in the journals and amongst knowledgeable farmers and entrepreneurs, a biochar industry never took hold. The reasons were certainly economic in the main: more than one enthusiast echoed the complaint made in the 1847 volume of the American Journal of Agriculture and Science: “The use of charcoal as a fertilizer is generally well known. Its expense, however, often precludes its use.”
Perhaps if Liebig or another scientist of his stature had advocated the true importance of soil carbon, a biochar industry might have developed. But Liebig never did discover that soils could become depleted of carbon. He was convinced that soil carbon would always be replenished by the action of plants taking carbon from the atmosphere: “A certain quantity of carbon is taken every year from the forest or meadow, in the form of wood or hay, and, in spite of this, the quantity of carbon in the soil augments; it becomes richer in humus.” (Agricultural Chemistry, p 14.)
Liebig did not live to see the widespread use of the ammonia fertilizers that have rapidly burned up soil carbon across the globe. Scientist Rattan Lal estimated in 2007 that “most agricultural soils have lost 30% to 75% of their antecedent soil organic carbon,” equivalent to 30 to 40 tons of carbon per hectare.
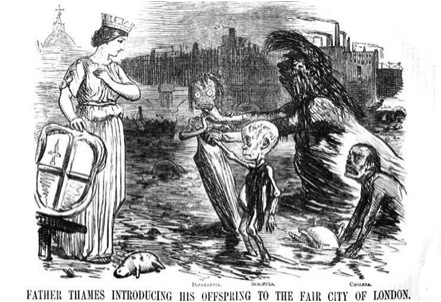 Figure 3. Cartoon depicting London's Great Stink of 1858: “In the summer of 1858, a combination of high temperatures and sewage overflow unleashed water-borne diseases and a malodorous stench on England's capital. This Punch magazine cartoon depicts ‘Father Thames’ introducing his offspring -- diphtheria, scrofula and cholera --to the City of London.”
Figure 3. Cartoon depicting London's Great Stink of 1858: “In the summer of 1858, a combination of high temperatures and sewage overflow unleashed water-borne diseases and a malodorous stench on England's capital. This Punch magazine cartoon depicts ‘Father Thames’ introducing his offspring -- diphtheria, scrofula and cholera --to the City of London.”
On the other hand, Liebig did observe that adding manure to promote plant growth could eventually deplete soils of other chemical nutrients. He cited the example of a vineyard at Bingen on the Rhine that was heavily manured, greatly increasing plant growth. But the effect did not last because the soil was soon depleted of potash, removed with the harvest and not returned to the soil by the particular manure used. Yields soon plummeted and were worse than before the fertilization. Here was the law of the minimum at work, with a twist. For the first time it was apparent that fertilizer could actually harm soil. (Agricultural Chemistry, pp 40-41.)
Liebig was very astute in another observation that was connected to some larger criticisms he had of the European system of agriculture. In contrast with China and Japan, where every bit of nutrient was returned to fields as human waste, Europe fertilized its fields with cattle droppings and dumped the waste from cities in rivers or other unproductive places. The problem was not just the loss of nutrients from sewage. Liebig realized that the cattle droppings came from pasture that would eventually become depleted because the deep roots of pasture grasses took minerals from the subsoil, and the minerals were transferred to the fields to grow grain that was exported as food to cities. Liebig put it this way: “The more fodder, the more flesh; the more flesh, the more manure; the more manure, the more grain.” (Letters on the Utilization of London Sewage.) While this practice might work for a long time, eventually, the subsoil would become exhausted of minerals and the animal manure would no longer suffice to fertilize the grain.
The Sewage Question
Liebig felt that the best long term solution to soil fertility was to adopt some form of the Chinese system. He said: “When human excrements are treated in a proper manner, so as to remove the moisture which they contain without permitting the escape of ammonia, they may be put into such a form as will allow them to be transported, even to great distances.” He acknowledged the efforts in many European cities to manufacture fertilizers from human waste but found that “the manner in which this is done is the most injudicious which could be conceived.”
This manufactured manure was known as “poudrette,” a French term meaning “crumbs” or “powder.” It was made from the contents of “dry closets” (as opposed to “water closets”) that were hauled to the outskirts of cities and mixed with various additives such as ashes, peat, gypsum, clay or lime. Depending on the admixtures and the process for incorporating them, the results were more or less valuable as a fertilizer. The main problem was the loss of nitrogen. Liebig found that “the greatest part of their urea is converted into carbonate of ammonia… and the vegetable matters contained in them putrefy; all their sulphates are decomposed… The mass when dried by exposure to the air has lost more than half of the nitrogen, which the excrements originally contained.”
Liebig recommended, “calcined mud and finely divided charcoal” (Agricultural Chemistry, p 66) as additives that would effectively retain the nitrogen. He advised manufacturer James Manning on his patented formulation (Brock, p 271) that included “soot, wood charcoal, seaweed charcoal, animal charcoal, or phosphate of lime, sulphates of manganese and of iron.”
But the industry was without regulation or standards. Ingredients might include coal tar, road sweepings and the wastewater from tan-yards, for instance. This material might be worse than ineffective. It might be toxic. Poudrette soon had such a bad reputation that farmers would not buy it.
Even when the poudrette was of good quality, farmers did not know it. The English town of Ely used large quantities of charcoal and gypsum in its manufactured manure, ensuring that it would retain useful quantities of nitrogen and other fertilizers, but farmers would not buy it because it had no “scent” – their way of determining the value of a fertilizer. They had no knowledge of the power of charcoal to deodorize and preserve nutrients and so they passed it by.
The alternative to dry toilets was some form of sewage or “water carriage” of excrement. While cities in Europe and America had long had drainage systems for storm water and household gray water, they were not intended for human waste, and many cities had laws against disposal of excreta in sewers. But the wealthy segment of society rapidly adopted the new water closets that piped their waste directly into the sewers, and by the 1840s, cholera was epidemic in London. It all came to a head in 1858, when a hot summer produced what was afterward called “the Great Stink” and thousands of people died from cholera. London shut its doors and windows, business ground to a halt and the government finally passed laws to come up with a plan to overhaul the London sewerage system, though it would be decades before a plan was devised.
It was in 1858 that Irish engineer, Jasper Wheeler Rogers, issued a broadside titled: “Facts and Fallacies of the Sewerage System of London, and Other Large Towns.” Rogers called the proliferation of water closets and the lack of any action to improve the situation, “the sin of the rich – sin emanating from selfish personal comfort solely, with utter forgetfulness of consequences to others.”
Rogers thought he had discovered a win-win-win system. Ever since reading Liebig’s report on the power of charcoal to absorb ammonia, he had been convinced that peat charcoal was the solution to the waste problem. Peat charcoal could also solve another great problem in his native Ireland: famine. In an earlier paper, “The Potato Truck System of Ireland: The Main Cause of Her Periodical Famines and of the Non-payment of Her Rents,” Rogers had exposed the true nature of the Irish famine. Ireland had food, but it was being sold elsewhere because in Ireland, potatoes were money. Laborers on the farming estates were paid in potatoes, not gold. When the potatoes rotted, their cash melted away. Rogers envisioned an industry that would pay cash wages for converting Ireland’s peat to charcoal that could then be used to deodorize the filthy sewers of Dublin, London and other towns. He would eliminate famine, clean up the sewers and restore soil fertility with his peat charcoal.
At a lecture in 1849 attended by 600 people, Rogers gave a demonstration, mixing peat charcoal with foul-smelling night soil. As a newspaper reported: “a few minutes before, all noses were turned away from the tin buckets in which the night-soil was brought; a few minutes after [it was mixed with peat charcoal], it was taken up in handsful and put into paper bags provided, and ‘stowed away,’ possibly in the same pocket with the pocket handkerchief.” A panel of appointed judges then signed a certificate: “We the undersigned… do unanimously decide as follows: That Mr. JW Rogers has fully proven the deodorising power of Peat Charcoal upon Human Excrement.”
As a result of his lobbying, the Irish Amelioration Society was established by Royal Charter in 1849, and in 1850, the Society opened a manufacturing center in Kildare County with a peat drying system and retorts to produce the peat charcoal, employing 300 people. The plan was to open a hundred such facilities throughout Ireland.
In 1851, Rogers exhibited his peat charcoal privy system at the Great Exhibition, but in 1853, Rogers found himself in debtor’s prison in London, his estates and effects confiscated. Still, by 1858 he was back promoting his ideas and issuing new patents, including one for a pneumatic sanitation system that would move the contents of dry closets and mix them with peat charcoal.
Rogers’ system never took hold. Perhaps the cost of manufacturing and transporting the peat charcoal to London was too high, or perhaps the convenience of the water closet was just too seductive. In 1876, the Local Government Board of London reported to Parliament with recommendations on sewage disposal methods and came down firmly on the side of hydraulic sewage systems. They concluded: “none of the manufactured manures made by manipulating town’s refuse with or without chemicals, pay the contingent costs of such modes of treatment.”
Conclusion
The interest in charcoal as a soil amendment nearly died out as agriculture became more and more reliant on cheap, easily applied chemical fertilizers. A few agronomists continued to investigate and write about soil charcoal for special uses like plant propagation, but little more was said until early in the 21st century when soil scientists discovered Terra Preta. Replenishment of soil carbon was taken up as a cause by the organic movement as early as the 1940s, but most farmers are still not aware of the need. The passion for recycling waste was revived in the 1970s and is receiving more attention now as municipalities struggle with burgeoning mounds of waste.
Liebig’s warnings about nutrient depletion have continued to resonate. His biographer William Shenstone wrote in 1895: “At present we are gradually wasting a capital which we ought to make increasingly valuable, and which no human power can restore once it has dissipated. The problem is not insoluble. It has been solved by races we are pleased to regard as almost barbarians. Till we, too, attain a solution suited to our conditions, we remain mere robbers and wastrels.”
The problem is still with us. Chemical fertilizers have postponed the day of reckoning, but we are much closer to the edge of disaster than we realize and have very little time left to craft the suitable solution.
The end came for Liebig in 1873. On his deathbed, he arranged his final experiment. He ordered his coffin and directed that “his body should be packed in charcoal and buried in Darmstadt.” (Brock, 327)
Our opportunity to build a biochar industry is just beginning. Let us follow the legacy of the great experimentalist, and do whatever we must to restore carbon to the soil.
References
Brock, W. H. (2002). Justus von Liebig: The chemical gatekeeper. Cambridge University Press.Campbell, D. (1858). On the application of sewage to agriculture. Quarterly Journal of the Chemical Society of London, 10(3), 272. doi:10.1039/qj8581000272
Krepp, F. C. (1867). The Sewage Question. Longman. London.
Lal, R., Follett, R. F., Stewart, B. A., & Kimble, J. M. (2007). Soil carbon sequestration to mitigate climate change and advance food security. Soil Science, 172(12), 943-956.
Lemon, J. (n.d.). The Great Stink. choleraandthethames.co.uk. Retrieved June 24, 2013, from http://www.choleraandthethames.co.uk/cholera-in-london/the-great-stink/
Liebig, J. (1852). Agricultural Chemistry, TB Peterson, Philadelphia.
Liebig, J. (1865). Letters on the Utilization of London Sewage.
Local Government Board (1876) Sewage Disposal: Report of a Committee. London: Her Majesty’s Stationery Office.
Merchant, C. (2010). Ecological Revolutions: Nature, Gender, and Science in New England. UNC Press.
Rogers, J. W. (1847). The Potato Truck System of Ireland: The Main Cause of Her Periodical Famines and of the Non-payment of Her Rents. London: James Ridgway.
Rogers, J. W. (1858). Facts and Fallacies of the Sewerage System of London, and Other Large Towns. London: Atchley.
Shenstone, W. A. (1895). Justus von Liebig: His Life and Work (1803-1873). London: Cassell.
Turner, S. (2012). The Garden Diary of Martha Turnbull, Mistress of Rosedown Plantation. Baton Rouge, LA: LSU Press.
Unknown (1847). Peat Charcoal. American Journal of Agriculture and Science, Volumes 5-6.
Unknown (1850, September 28). The Irish Amelioration Society Manufacture of Peat Charcoal. The Illustrated London News.
Unknown (1853). Manures. New York State Agricultural Society, 12 (112) 867.
Unknown (1856, September). Charcoal as a fertilizer. The New Jersey Farmer, 2(1), 75.
Unknown (1873, October 11). Charcoal as a fertilizer. The Garden: an illustrated weekly journal of gardening in all its branches, 4, 307.
Wilder, M. P. (1851, July). On the use of charcoal. The Journal of Agriculture, 14-15.


A simple observation
I found this write-up on Justus Liebig very informative and fascinating. His experiments with pure charcoal in curing sickly plants was interesting. Even if plant growth were not as well understood then as it is today - there's no doubting his observations and findings.
I too would like to share an observation I made regarding the laying bare of charcoal over a prolonged period of time. I was busy setting up a tent for Biochar storage when I noticed a pile of charcoal 'fines' of around 3 tons (under 15 mm diameter) was still there two years on following a charcoal project we did there. The 'fines' were dumped in a few spots continuously between November 2011 to April 2012. There is a demand for fine charcoal by the lucrative briquetting industry, but they only pay around $40 per ton. This farm is frequented by cattle, with dung piles littered over it, but no fires have been through it in this specific area. Have a look to this picture refers: http://vuthisa-techblog.com/wp-content/uploads/2014/12/Biochar_observation_smaller.jpg: the area circled in red is the same area in the picture inlay directly above it – but 20 months later. The charcoal layer somehow compacted down on top of the grass cover and is only reached by digging down 0.5 metres or so.
Another interesting development was the appearance of natural vegetation somehow anchored to the charcoal. See this picture showing the plant growth: http://vuthisa-techblog.com/wp-content/uploads/2014/12/Plant-growth.jpg I guess what really intrigues me is how it is possible for charcoal to compact in this manner? what are the advantages of laying it bare? And would this practice improve the conditioning of the char, i.e. load the char with beneficial microbes? The fact that natural vegetation have taken root on it indicates to me that the biochar is already inoculated to some degree. The char dust particles would no doubt have filtrated down by rainfall, but I suspect the larger chunkier pieces would have remained in place more or less.
For those interested in how this charcoal was made and note that I do not refer to it as biochar - we used a metal TPI kiln in a ‘direct combustion’ method whereby the heat for carbonization comes from burning a portion of the biomass feedstock in a limited air environment. At that stage (2011/2012) our mission was to clear invasive plant species and convert them into char and not to produce a soil amendment product. Having said that, there is little difference between the TPI kiln and the Moxham kiln used for producing Biochar. We have since then incorporated 3 x 55 gal drum retorts into the design to produce Biochar. I received a phone call the other day from a local commercial sugarcane farmer desperate to improve his soils. He has seen an annual decline in his yields and he knows that his soils need a long term remedial plan - something fertilizers don't offer. We've decided to do some plant trials on his farm. If a 15 minute phone conversation with a relative newcomer like myself can convince a seasoned farmer that Biochar MUST be the only way to go, I can't imagine the educational impact of a field day or just an Internet presence on attendees/site visitors. No matter how small and isolated our own experiments seem to be, we CAN make a contribution - and perhaps it starts with simple observations.