Technical Abstract
Biochar land application research in elevated rainfall areas (980 mm annual rainfall) of the U.S. Pacific Northwest is lacking. A proof-of-concept field study examined the effects of spruce-pine-fir wood chip biochar (slow pyrolysis; 450-500 oC; 35 Mg ha-1), dairy manure compost (105 Mg ha-1), compost + biochar (35 and 105 Mg ha-1, respectively), and a control (no biochar or compost) on glacially altered soil chemical properties and growth characteristics of vetch (Vicia spp.) and sweet corn (Zea mays L. Golden Jubilee) over a growing season. In-season liming (5.4 Mg ha-1) occurred across all the plots to raise the soil pH for adequate crop growth. Biochar, alone or applied with compost, maintained a greater amount of soil organic C and, when combined with lime, acted more effectively than control conditions at increasing soil organic C. Biochar and compost + biochar treatments reduced Mehlich-III extractable Zn and Cu concentrations, although the concentrations were an order of magnitude greater than those considered minimal for crop growth. There was no statistical difference in vetch or corn yields among treatments. However, the compost + biochar treatment did increase vetch total N and Mg content, as well as corn Cu content, as compared to other treatments. Overall, observations suggest that co-applying biochar with an organically-rich material like compost could be beneficial without compromising environmental quality.
The SeaChar Field Trial
Our project was initiated by the Seattle Biochar Working Group (SeaChar), founded in Seattle Washington in 2008 by two area residents (metal-work artists Art Donnelly and Don Hennick) and many volunteers (including Jim Grob, James Whittaker, Sue Dickson, and Steve Tracy). The purpose and motivation of SeaChar was to test the effects of biochar on local soils and to involve volunteers in a citizen science project that would educate students and others about soils and biochar and, if the results showed promise, use them to promote biochar for urban farming and local food production.


Left: SeaChar founders Don Hennick and Art Donnelly with a TLUD stove they designed. Right: Informational placard at the SeaChar Carbon Garden at South Seattle Community College.
In 2009, SeaChar volunteers reached out to the campus of South Seattle Community College, Seattle, Washington, and a research location was secured. Much of the outreach for working with campus administration was facilitated by SeaChar board members Vivian Scott and Anita Hornby. The approximately 0.10 ha site was located between a parking lot and a fence at the south end of campus, largely covered in blackberry bramble cleared prior to site establishment. Soil on the site was likely composed of a mixture of native soil along with fill removed during parking lot construction.


Volunteers carefully apply compost, lime and biochar to the various blocks.
Following site selection, Seachar volunteers reached out to soil science and biochar experts from both academia and the biochar technical community. The experts responded and provided guidance on plot management and treatment amounts. However, many of the experts were a long distance away and thus were not able to visit the site in person. The project utilized four treatments replicated twice in the field in a randomized complete block design (i.e. all treatments applied to specific plots, established at random, within two blocks). Blocking treatments is to account for on-site variability, dividing blocks into relatively homogenous subgroups or blocks and laying out the different treatments in a random fashion within those blocks. The variance in the data is theoretically reduced and thus the study can focus on differences between treatments instead of differences between blocks. (see http://www.biochar-international.org/sites/default/files/IBI_Biochar_Trial_Guide_final.pdf).
Prior to site establishment, the location was an untended blackberry bramble that needed to be cleared. A one-time treatment application was utilized as suggested by Dr. Julie Major, as this approach was consistent with other research approaches worldwide. This approach would allow for comparisons to be made between our project and others. In addition to biochar, compost was used as a treatment as this had been advocated by others (e.g., Dr. Bruno Glaser from Germany) for a systems approach to organic amendment comparisons (e.g., compost vs. biochar). During the project, we were fortunate to enlist the help of Mr. Jim Grob to collect soil and plant samples on a relatively routine basis. Finally, we intended to conduct the research for a minimum of three harvests, and had a five-year lease with the College. However, we completed the work only after two field seasons and one harvest due to an in-field blocking error that was beyond our control after plot establishment (see the Challenges to Community-Based Research section, below).
Background
Social objectives
The SeaChar project had both social and scientific objectives. One important social objective was to counteract negative perceptions of biochar that SeaChar volunteers had encountered in their efforts to promote the use of charcoal as a soil amendment. Despite the apparent success of biochar use in tropical soils, some gardeners worried that the effects of biochar in their soils could be detrimental. SeaChar wished to show that biochar was at least not detrimental so that local gardeners could feel confident that it was worthwhile to try in their own soils. SeaChar also wanted to involve students and volunteers in learning more about scientific approaches to soils and urban farming. Finally, SeaChar wished to establish the test plots in a publically accessible area so that the general public could observe differences, if any, between soils amended with biochar and the alternative treatments.


Volunteers of all ages took part. Left: Elementary students mulched the paths. Right: The Seattle Youth Corps pitched in to pull weeds.
Science objectives
The scientific objectives of the SeaChar field trial were primarily to address a knowledge gap in biochar research: few, if any, studies have been performed regarding biochar effects on the younger, relatively unweathered soils of the cool, humid Pacific Northwest. Our objective was to, via a proof of concept study, determine the effects of a one-time, realistic biochar or compost application, or a biochar and compost co-application, on soil, vetch (Vicia spp.) and sweet corn characteristics near Seattle, Washington, USA.
Increases in the soil nutrient status have been observed in relatively unweathered to highly weathered soil systems (Table 1). Biochar application may increase available nutrients across a wide array of soil types because biochar-associated elements may be present as soluble salts (Cao and Harris, 2010; Knicker, 2007) or selectively sorbed on exchange sites (Ippolito et al., 2012a; Novak et al., 2009a; Namgay et al., 2010), both of which could provide nutrients to the soil solution and thereby to plants. Thus, biochar may act similarly to a fertilizer for some nutrients as presented in Table 1, yet it must be kept in mind that biochar would be equal to a relatively low grade fertilizer.


Left: Observing different growth patterns. Right: Grinding corn stalks for analysis.
|
Soil System |
Biochar Application Rate |
Observed Change in Soil Nutrient Status |
Reference |
|
Arid; relatively unweathered |
Up to 100 tons/ac (up to 224 Mg/ha) |
Increases in plant-available Fe, Mn, and Zn
|
Ippolito et al. (2014b); Ippolito et al. (2014c) |
|
Arid; relatively unweathered |
20 tons/ac (45 Mg/ha) |
Increase in plant-available Mn
|
Lentz and Ippolito (2012) |
|
Arid; |
20 tons/ac (45 Mg/ha) |
Increases in plant-available Mn, Ni, and K
|
Ippolito et al. (2012a) |
|
Semi-arid; relatively unweathered |
5 tons/ac (12 Mg/ha) |
Increases in plant-available P, K, and Fe
|
Brewer et al. (2012) |
|
Semi-arid; relatively unweathered |
~10 tons/ac (~20 Mg/ha) |
Increases in plant-available Ca, Mg, K, and P
|
Laird et al. (2010) |
|
Humid; relatively weathered |
10 tons/ac (22 Mg/ha) |
Increases in plant-available K
|
Gaskin et al. (2010) |
|
Tropical; highly weathered |
100 tons/ac (224 Mg/ha) |
Increases in plant-available K |
Lehmann et al. (2003) |
Table 1. Positive changes in soil nutrient status in relatively unweathered to highly weathered soil systems following biochar application, as compared to soils that did not receive biochar.
However, increases in plant-available nutrients do not always occur. In some instances decreases occur, while in others situations no change occurs as illustrated in Table 2.
|
Soil System |
Biochar Application Rate |
Observed Change in Soil Nutrient Status |
Reference |
|
Arid; relatively unweathered |
4 tons/ac (10 Mg/ha) |
No change in plant-available nutrients
|
Van Zwieten et al. (2010) |
|
|
|
|
|
|
Arid; relatively unweathered |
20 tons/ac (45 Mg/ha) |
Decrease in plant-available P
|
Ippolito et al. (2012a) |
|
Arid; relatively unweathered |
Up to 100 tons/ac (up to 224 Mg/ha) |
Decreases in plant-available NO3-N
|
Ippolito et al. (2014a); Ippolito et al. (2014b); Ippolito et al. (2014c)
|
|
Arid; relatively unweathered
|
4 tons/ac (10 Mg/ha) |
No change in plant-available NO3-N within 4 months; 75% reduction in NO3-N after one year
|
Ventura et al. (2013) |
|
Humid; relatively weathered |
Up to 40 tons/ac (up to 90 Mg/ha) |
Decrease in plant-available P
|
Parvage et al. (2013) |
|
Humid; relatively weathered
|
Up to 35 tons/ac (up to 80 Mg/ha) |
Decreases in plant-available P, K, S |
Hass et al. (2012) |
|
Tropical; highly weathered |
100 tons/ac (224 Mg/ha) |
No change in plant-available nutrients |
Lehmann et al. (2003) |
Table 2. Negative or neutral changes in soil nutrient status in relatively unweathered to highly weathered soil systems following biochar application, as compared to soils that did not receive biochar.
Soil and plant responses to biochar application have been related to changes in soil fertility, quantity of initial available soil N, and biochar chemical characteristics (Spokas et al., 2012). Thus, there are a plethora of variables that determine plant response to biochar application. More specifically, these include biochar characteristics (e.g., available nutrients, pH, ability to attract or repel water, etc.), biochar application rate and the immediate effect on soil water relations and microbiological activity, the initial soil fertility status, and plants to be grown. The aim of the current study was to identify some of the variables affecting plant growth when utilizing biochar. Compost was also utilized in the study since it is a readily available organic source of nutrients, used by many homeowners, and at the time of study establishment was being suggested as a comparison material to biochar.
Materials and Methods
Biochar and Compost Characterization
The biochar, supplied by Alterna Biocarbon (Prince George, British Columbia, Canada), was created from a spruce-pine-fir wood chip feedstock mixture using slow pyrolysis at a temperature of 450-500 oC. Dairy manure compost was supplied by Bailey Compost (Snohomish, WA). Biochar and compost chemical characteristics are presented in Table 3.
|
Property |
Units |
Biochar |
Compost |
Everett/Alderwood soil |
|
pH |
|
6.8 |
6.8 |
5.2 |
|
EC |
dS m-1 |
0.1 |
3.3 |
0.2 |
|
Total C |
% |
67.4 |
25.2 |
2.80 |
|
Total N |
% |
0.24 |
1.27 |
0.23 |
|
Organic N |
% |
0.24 |
1.25 |
0.23 |
|
NO3-N |
mg kg-1 |
1.60 |
167 |
28.2 |
|
NH4-N |
mg kg-1 |
0.60 |
26.2 |
5.52 |
|
K |
mg kg-1 |
1600 |
4620 |
60.8 |
|
Ca |
mg kg-1 |
3890 |
7260 |
854 |
|
Mg |
mg kg-1 |
2790 |
4590 |
204 |
|
Na |
mg kg-1 |
347 |
392 |
ND‡ |
|
P |
mg kg-1 |
2650 |
1390 |
193 |
|
Al |
mg kg-1 |
2380 |
5880 |
ND |
|
Fe |
mg kg-1 |
3740 |
9610 |
323 |
|
Zn |
mg kg-1 |
62.7 |
45.0 |
48.9 |
|
Mn |
mg kg-1 |
185 |
244 |
17.0 |
|
Cu |
mg kg-1 |
20.3 |
26.5 |
28.6 |
|
Ni |
mg kg-1 |
3.6 |
11.1 |
ND |
|
Mo |
mg kg-1 |
0.84 |
<0.05 |
0.06 |
|
Cd |
mg kg-1 |
0.54 |
0.32 |
ND |
|
Pb |
mg kg-1 |
0.5 |
9.7 |
ND |
Table 3. Properties and total elemental analysis of the biochar and compost, and properties and Mehlich-III elemental analysis of the soil.Analyses are described in the appendix. ND = not determined.
Experimental Setup and Design
The experimental site was located on the South Seattle Community College Campus, Seattle, WA (lat. 43o 32’ 41.98” N, long. 122o 21’ 8.35” W; elevation 100 m; 980 mm annual rainfall), and was established by a group of aforementioned volunteers with the guidance of a research team. Soils at the research site were classified by the USDA-Natural Resource Conservation Service as Inceptisols and were part of the Everett (sandy-skeletal, isotic, mesic Humic Dystrochrepts) and Alderwood (loamy-skeletal, isotic, mesic Aquic Dystrochrepts) soil series (University of California, Davis Soil Resource Laboratory, 2008). Inceptisols are relatively young soils and are not weathered to a great extent. Their productivity can vary widely depending on location. Inceptisols in the US Pacific Northwest are typically quite fertile (Brady and Weil, 1999). Background soil characteristics are presented in Table 1 above.
We chose to follow analysis similar to other biochar research projects at the time the study was established. We evaluated changes in the soil by measuring pH, EC (electrical conductivity), nutrients and carbon content. We analyzed impacts on the plants by measuring plant growth and plant nutrient content.
In June 2009 and prior to treatment application, eight 4.5 m x 4.5 m plots were established. Four amendment treatments included the following: 1) control (no biochar or compost application), 2) composted manure applied at 105 Mg ha-1 (dry wt.), 3) biochar applied at 35 Mg ha-1 (dry wt.) application, and 4) composted manure + biochar co-application using rates identical to manure-only and biochar-only treatments. Figure 1 illustrates the complete block design with two replicates of all treatments within each block. This experimental design is utilized to account for and reduce in-field variability. In our case, there was in-field variability from block 1 to block 2. Thus, within each block the in-field variability is reduced, and all treatments studied (in our case, four) are placed, at random, within the block. With this experimental setup, differences between treatments can be discerned without the effect of in-field variability.
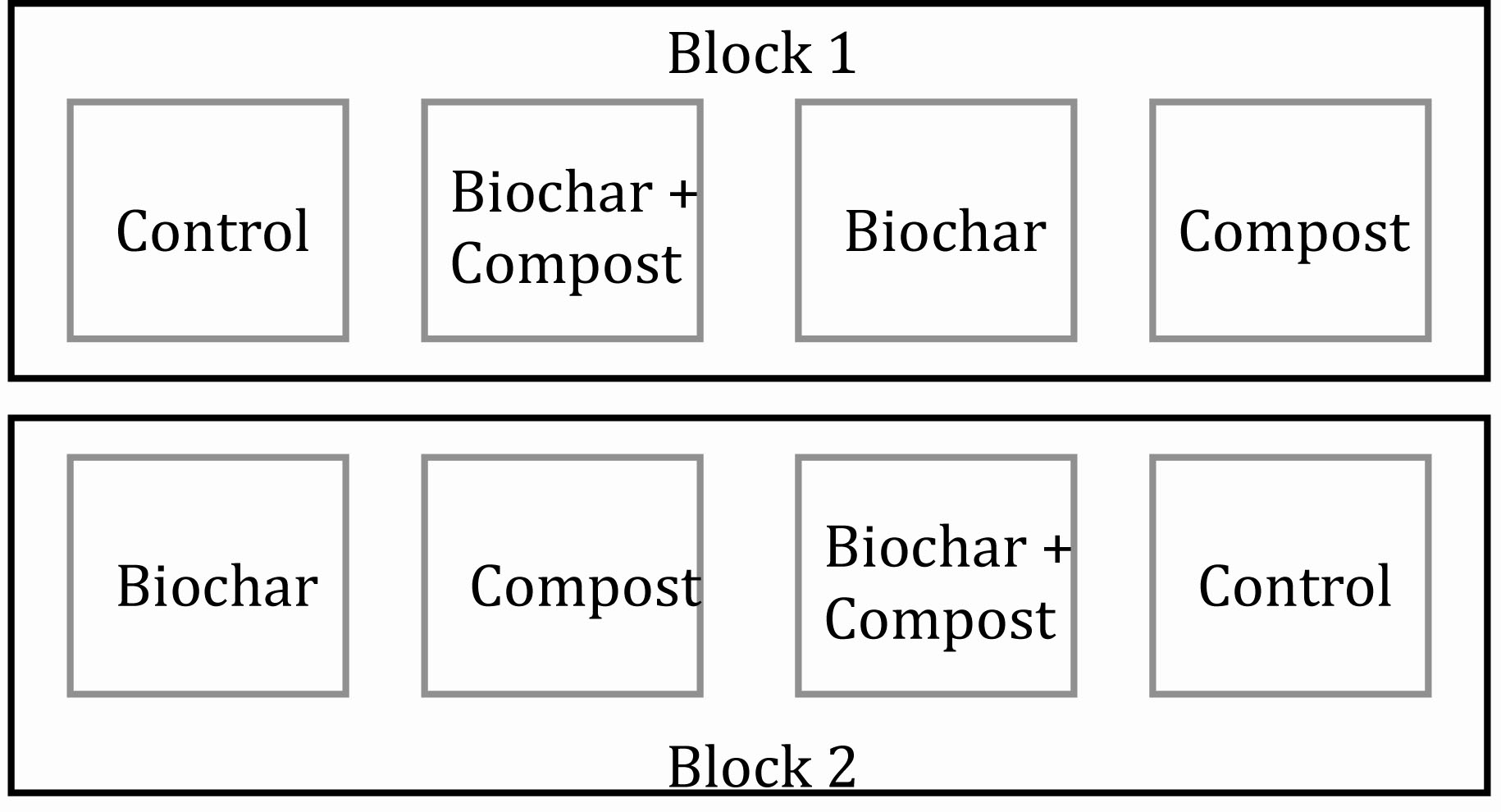
Figure 1. Experimental plot setup using a randomized complete block design with two replicates.
Treatments were hand-applied in July 2009, and all treatments were rototilled into the soil to a depth of 10 to 15 cm. A one-meter, unplanted border separated all plots to help keep plots separate from one another. In October 2009 all plots were seeded with vetch. The vetch was grown in order to potentially improve soil N dynamics. The vetch was mowed to a height of ~ 10 cm on May 14, 2010; clippings were collected, weighed for yield determination, and analyzed for nutrient content. Analyses are described in the appendix.
Following vetch collection, lime was hand-applied to all plots at a rate of 5.4 Mg ha-1 to increase the initial soil pH (5.2) to optimal for corn growth (pH 5.8 to 6.2; Hart et al., 2010). Then, the remaining vetch and lime were incorporated to a depth of ~ 10 cm with a rototiller. An additional soil sample was obtained on May 21 to ascertain the liming effect, and all soil analyses were performed as previously noted.
All plots were hand planted with sweet corn (Zea mays L. Golden Jubilee) on June 5. We chose sweet corn because it grows readily in this environment and is relatively easy to cultivate and tend. In addition, both the USDA-Agricultural Research Service and Washington State University Extension services were also using corn or sweet corn in their biochar plot testing. Volunteers routinely monitored the soils over the growing season by collecting samples from all plots to a depth of 0 to 30 cm. Whole corn plants were harvested on Sept. 29 by removing plants within a 4.2 m2 area within each plot. The precise harvested area needed to be known in order to convert yield from such a small area to units known by most corn producers (e.g., megagrams of corn per hectare; Mg/ha = metric tons per hectare). The corn was weighed and then the ears were removed, counted, and weighed. Corn plants without ears were chopped and a subsample sent to the laboratory for nutrient analysis. Analyses are described in the appendix.
Statistical Analysis
Statistical analysis was performed on all soil and plant data to test whether or not differences existed between the observed averages of treatments. In our case, we tested whether or not differences existed between the observed averages from the control, biochar, compost, or biochar+compost treatments for the measured soil and plant constituents. For plants, we used a statistical method called analysis of variance to test whether or not differences existed between treatments. This statistical approach is used to compare the averages (and the variability of those averages) of more than two treatments. For the soils, we used a statistical method called “split with time” because this type of design tests whether or not differences existed between treatments, or within a given time period, or if differences existed both with treatment and time. For instance: we may be interested in knowing whether one treatment increases a soil nutrient as compared to other treatments (exactly like how we statistically analyzed the plant data); at the same time we may want to know if time affects a soil nutrient; and we may be interested in how the treatment and time combine to affect a nutrient (e.g., one treatment could increase a soil nutrient over time while another treatment could decrease a soil nutrient over time. This is called the interaction. We did not observe any significant interactions in this study).
We used a 95% confidence interval for all statistical analyses. This means that when differences existed between treatments, we were 95% confident that they really did exist. If significant differences were present between treatments, we then calculated a number called the Least Significant Difference (LSD; Steel and Torrie, 1980). The Least Significant Difference number indicates what value is needed to see a significant difference between average values of treatments, again with 95% confidence.
Results and Discussion
We analyzed the soils for organic C, total C, total N, NH4-N, NO3-N, pH, EC, and plant-available Ca, Cu, Fe, K, Mg, Mn, Mo, P, and Zn. We analyzed the vetch and corn for yield and for Al, Ca, Cu, Fe, K, Mg, Mn, Mo, Na, P, S, Zn, C, and N content. Only the significant results are presented below.
What Happened to the Soil ?
Organic Carbon
The biochar and compost + biochar treatments contained a greater percentage of soil organic C as compared to the control (Fig. 2a). The biochar and compost used in the study contained 67.4% and 25.2% C, respectively. Given the biochar and compost application rates of 35 and 105 Mg ha-1, and the initial soil organic C content of 2.80%, the biochar, compost, and biochar + compost applications should have increased the soil organic content by 1.39, 1.43, and 1.82 times, respectively. These estimated increases would equate to 3.92, 4.00, and 5.10% organic C per treatment, which are very comparable to values shown in Fig. 2a. Similar results have been observed in other studies (see Ippolito et al., 2014b, c). Increasing organic C content in soils is typically linked with improving the ability of soil to retain nutrients and moisture, and thus likely improving plant productivity. If the organic soil carbon content increases continues over a longer time scale due to improvements in nutrients, water, and plant productivity, the carbon sequestration potential through biochar soil amendment could much exceed the amount of carbon sequestered in biochar alone and could thus potentially become an important climate mitigation strategy.
The effect of biochar and compost + biochar treatments in elevating soil C was maintained over the study period, which could have been due to the cool site conditions limiting microbial degradation of the organic C sources. However, this result also supports the findings of Lentz and Ippolito (2012) that biochar may be recalcitrant and help protect compost from further microbial degradation. Indeed, recent research (Lentz et al., 2014) suggested that biochar may reduce soil microbial processes that affect organic matter degradation, thus leading to maintenance and accumulation of C from other sources such as compost. Opposite to these findings, Wardle et al. (2008) and Hamer et al. (2004) suggested that decreases in soil organic C content may occur because some biochar C may be degraded by microorganisms or that biochar stimulated microorganisms to degrade native soil organic C.
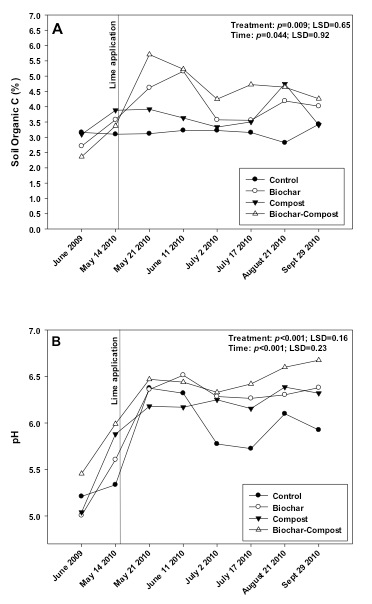
Figure 2. Changes in soil A) organic C content and B) pH over the study period in control (no added amendment), biochar (35 Mg ha-1), composted manure (105 Mg ha-1), and biochar + compost (35 and 105 Mg ha-1, respectively) amended soils.
pH
The biochar, compost, and compost + biochar treatments all had a slight liming effect, compared to the control, as the soil pH significantly increased from application date (June 2009) to the following spring (5/14/10; Fig. 2b). This was caused by both materials having a pH of 6.8 as compared to the soil pH of 5.2 (Table 3). Following lime application (5/14/10), the soil pH in all treatments increased as expected. However, the compost + biochar treatment showed the greatest pH shift over time, and maintained a greater soil pH as compared to all other treatments including the control. These observations were likely due to both biochar and compost containing some buffering capacity, or their ability to prevent the soil pH from decreasing.
Compost has been shown to increase the pH of acidic soils (Alvarenga et al., 2008, 2009) and thus can be an effective liming agent. Because of its neutral to basic pH, biochar may also be used as a liming agent (Kloss et al., 2012), to reduce soil acidity (Yuan and Xu, 2011; Uchimiya et al., 2012). The pH of biochar is strongly influenced by pyrolysis temperature. Enders and Lehmann (2012) observed an increase in pH of several biochars (cow manure, annual biomass, woody biomass) when pyrolysis temperature increased from 300 to 600oC. Increasing pyrolysis temperature typically removes acidic organic functional groups and causes biochar to become more basic in pH (Novak et al., 2009b; Li et al., 2002; Ahmad et al., 2012; Cantrell et al., 2012)and causes the nutrients to be in metal oxide, hydroxide, or carbonate form, resulting in elevated pH values (Cao and Harris, 2010; Knicker, 2007).
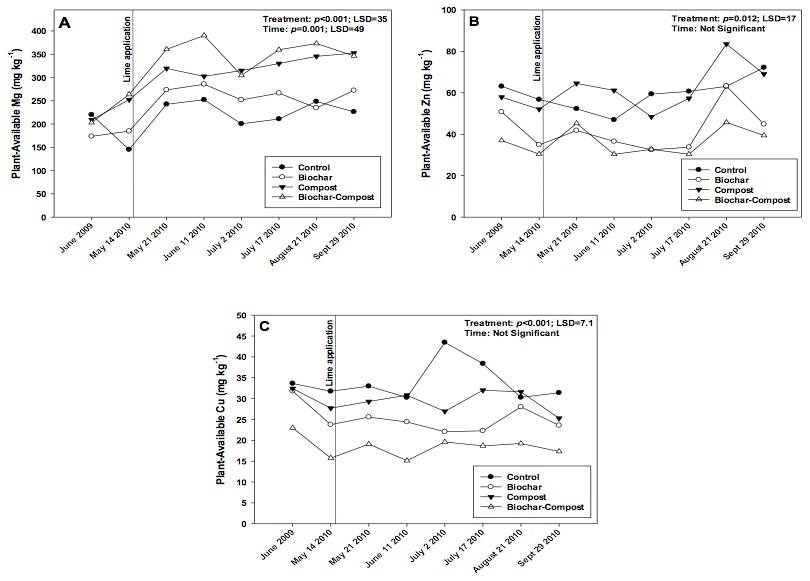
Figure 3. Changes in plant-available (i.e. Mehlich-III extractable) soil A) Mg, B) Zn, and C) Cu over the study period in control (no added amendment), biochar (35 Mg ha-1), composted manure (105 Mg ha-1), and biochar + compost (35 and 105 Mg ha-1, respectively) amended soils.
Plant-Available (i.e., Mehlich-III Extractable) Mg
Plant-available Mg increased over the course of the study (Fig. 3a), likely due to a combination of compost and lime application. The compost and compost + biochar treatments contained greater extractable Mg as compared to the control or biochar alone treatments. In addition, the concentration of extractable Mg increased following lime addition on May 14. The Mg concentration could have increased due to the presence of Mg in lime, or because the soil pH increased and subsequently increased Mg availability (e.g., see Whiting et al., 2011). Although we did not measure the Mg content in lime, in either case, increasing Mg availability could be construed as a positive in terms of plant growth.
Plant-Available (i.e., Mehlich-III Extractable) Zn and Cu
The biochar and compost + biochar treatments reduced plant-available soil Zn and Cu concentrations, likely due to biochar sorbing and making Zn and Cu partially unavailable for plants (Figs. 3b and c). However, plant-available Zn and Cu concentrations in all treatments were at least an order of magnitude greater than concentrations considered low for crops (1.6 to 3.0 mg Zn kg-1 soil, and < 10.0 mg Cu kg-1 soil; Espinoza et al., 2006). Biochar may retain nutrients via several mechanisms including entrapment of dissolved nutrients in water (Lehmann et al., 2003), surface sorption via surface groups, and electrostatic adsorption (i.e. cation exchange capacity; CEC). In the current study, biochar CEC could develop during pyrolysis or when the product was exposed to air and water, creating oxygenated surface functional groups (Briggs et al., 2012; Chan and Xu, 2009). Additionally, Cu and Zn may be physically trapped in internal biochar pores.
However, it was more likely that adsorption of Cu and Zn occurred on biochar. Beesley and Marmiroli (2011) observed a significant decrease in leachate Zn content from soil (~ 300 mg L-1) when leachate was subsequently passed through a biochar matrix (~ 10 mg L-1). The authors speculated that Zn was sorbed on outer surfaces of biochar, and when those sites were saturated with Zn, Zn sorbed onto inner pore surfaces. Sorption of Cu by biochar has been researched to a slightly greater extent. Borchard et al. (2012) suggested that oxygen-containing functional groups present in biochar are responsible for overall sorption. The authors found that Cu interacted chemically with biochar and physical interaction (i.e., entrapment) was negligible. Ippolito et al. (2012b) showed that, in part, Cu was bound to biochar via reactive organic functional groups on the biochar surface. Uchimiya et al. (2012) showed that by removing these functional groups from biochar, the retention of elements such as Cu were also reduced.
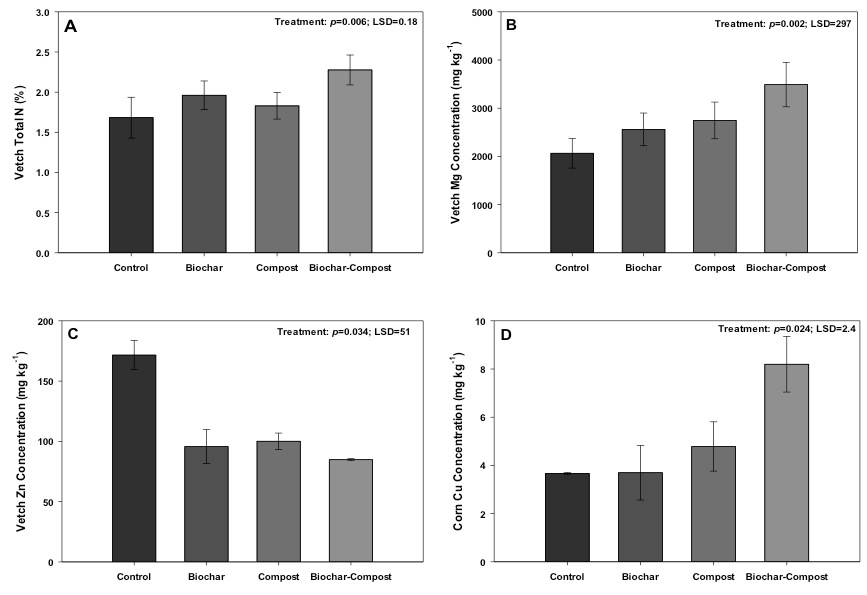
Figure 4. Changes in vetch A) total N, B) Mg, C) Zn, and corn D) Cu concentrations grown in control (no added amendment), biochar (35 Mg ha-1), composted manure (105 Mg ha-1), and biochar + compost (35 and 105 Mg ha-1, respectively) amended soils.
What Happened to the Plants?
Vetch Biomass
Vetch biomass did not significantly vary across treatments. Biomass for the control, biochar, compost, and biochar-compost treatments were 2.2, 1.9, 2.3, and 2.1 Mg ha-1, respectively. There was no significance difference observed across the treatments.
Vetch Total N
Co-applying compost and biochar caused a statistically positive synergistic effect in vetch total N content as compared to applying both materials separately (Fig. 4). This observation could be related to biochar leading to more efficient compost- or soil-borne N use by vetch, or by potentially increasing microorganisms involved with N fixation as suggested by Ducey et al. (2013). In support of this hypothesis, two growing seasons following application of biochar (22 Mg ha-1), manure (42 Mg ha-1), or biochar-manure at the same rates, Lentz and Ippolito (2012) observed an increase in corn silage total N uptake in biochar-manure treatments as compared to the biochar or control treatments. Lentz et al. (2014) explained the increase in N uptake as biochar maximizing net N mineralization in the presence of manure; mineralization is the conversion of organic N sources to NH4-N by microorganisms, with NH4-N a form of N available for plant uptake.
Similarly, Kammann et al. (2011) observed greater N use efficiency (e.g., the ability of a plant to use more N that is present in the soil) in quinoa (Chenopodium quinoa Willd.) when grown in the presence of biochar (100 and 200 Mg ha-1) and N fertilizer (100 kg ha-1) as compared to N fertilizer only. Chan et al. (2007) also observed a similar response in a biochar (0, 10, 50, and 100 mg ha-1) and N fertilizer study (100 kg ha-1).
Vetch Total Mg and Zn
The vetch grown in compost and compost + biochar treatments contained greater Mg as compared to the control (Fig. 4b), similar to the soil results. This result may be important because improving Mg content in plants such as vetch could help reduce the incidence of grass tetany in areas like western Washington State (Hart et al., 2009). Grass tetany is a disease in ruminant livestock such as beef and dairy, and involves a Mg deficiency leading to symptoms such as irritability, muscle twitching, staggering, collapse, coma, or death.
In addition, the vetch grown in biochar and compost + biochar contained less Zn as compared to the control (Fig. 4c). Vetch grown in the compost treatment also contained less Zn than the control, which was unexplainable because Mehlich-III extractable Zn concentrations were similar between the control and compost treatment early in the study.
Corn Biomass
Similar to the vetch biomass findings, corn total biomass did not significantly vary across treatments. Biomass for the control, biochar, compost, and biochar-compost treatments were 4.8, 4.8, 4.6, and 5.1 Mg ha-1, respectively. The number of corn ears and corn ear weight also did not vary significantly among treatments.
Corn Cu
The compost + biochar treatment increased corn Cu concentration over the other treatments (Fig. 4d), although all treatments contained adequate Cu levels (3 mg kg-1; Fageria, 2001). The increase in corn Cu concentration in the compost + biochar treatment was surprising, and unexplainable, because this treatment contained the lowest concentration of Mehlich-III extractable Cu over the course of the study (Fig. 3C). Namgay et al. (2010) showed an opposite response, whereby corn shoot Cu content was reduced by biochar application. The authors attributed the reduction due to Cu sorption by biochar, while Ippolito et al. (2012b) proved that biochar can sorb Cu onto either the surface or precipitate Cu as a Cu-carbonate or Cu-oxide mineral within the biochar matrix. Regardless, the compost + biochar treated corn Cu content was similar to that found by Moore et al. (2014) in a survey of 39 producer fields, while the control, biochar, and compost only treatments were lower than concentrations found by Moore et al. (2014). This suggests that the compost + biochar treatment may be beneficial in terms of supplying Cu to the plant even when soil extractable Cu concentrations are low.
Social Impacts
The SeaChar project successfully engaged a number of students from South Seattle Community College in conducting the field trial. Community colleges serve a widely diverse group of students, which allowed SeaChar to reach a more diverse segment of the public than might have been possible at another location. In addition to the educational impact of directly involving students in plot establishment and tending, SeaChar brought additional groups on campus to help with weeding the plots, including several groups of elementary grade and high school students. The test plot became part of the campus Earth Day event and was a focus of interest for a Permaculture Convergence event. SeaChar was satisfied that the test plot results were sufficient to reassure local gardeners that biochar would not harm soils and that it would be safe to experiment with biochar for potential soil improvement and soil carbon sequestration.
Challenges to Community-Based Research
The current in-field project initially began with four blocks, not two. We had good intentions at the onset but did not, initially, properly account for site variability. Instead of blocking with site variability, blocks were unfortunately established across site variability (i.e. vertically instead of horizontally as in Figure 1). In Figure 1, a parking lot was adjacent to Block 2, and soils in block 2 were likely affected to a greater degree (as compared to Block 1) due to disturbance from parking lot development. Our error in the initial blocking mistake was only noticed after data were statistically analyzed long after plot establishment.
Another challenge of working in a community college environment is the high turnover of students who may only be on campus for one or two years. Faculty and staff have full schedules and may not find it easy to devote sufficient time to adequately supervise a long-term field trial, despite initial assurances. While the project received advice and support from biochar researchers outside the area, in retrospect, it would have been best to involve a principle investigator who was able to visit the site location before plot establishment to make a better determination of an optimal design layout, including a more thorough background soil collection and analysis prior to plot establishment.
Based on our experiences we strongly advise that community based groups that wish to evaluate biochar recruit the assistance of a local scientist as lead investigator to guide the study. We were fortunate enough to re-block the treatments into two blocks (due to sheer luck of how we initially set up the study) and salvage a portion of our study; others may not be as fortunate.
Summary
Biochar application, either alone or in tandem with compost, provided an increased and sustained over-the-growing-season amount of soil organic C as well as acted like a liming agent to maintain a more optimal soil pH for crop growth as compared to the control soil. This observation suggests that the pH effect of biochar and lime co-application may be maintained for a longer period of time as compared to lime application alone, and thus may provide a long-term cost savings to producers. Biochar and biochar + compost applications did result in lower extractable soil Zn and Cu, but nutrient concentrations remained an order of magnitude greater than minimum concentrations for crop growth.
In this study, plant growth did not suffer due to biochar application. In addition to these positive attributes, the compost + biochar treatment increased the total N and Mg content in vetch and improved corn Cu content over other treatments. The improvement in vetch Mg content alone, associated with biochar-compost co-application, and may improve forage quality in areas prone to grass tetany issues. Thus, the co-application of compost + biochar could be more beneficial than biochar alone for maintaining or improving plant and soil quality
Future Work
The research site could potentially be utilized in the future to track long-term changes in soil characteristics as well as plant responses, similar to the constituents measured in this study. Perhaps such detailed analyses would not have to occur, and plants and soils could be monitored once yearly or bi-yearly at harvest. In addition, it would be interesting to measure changes in the soil microbial community status, since microorganisms play a major role in N transformations, organic C degradation, and cycling of other macro- and micro-nutrients. Additional, future on-site research could also include a re-application of all treatments as this approach is not often followed in research programs world-wide. Future, community based studies with regards to replicated field trials or more simple demonstration experiments could very well support this approach in terms of local ownership.
References
Ahmad, M., S.S. Lee, X. Dou, D. Mohan, J. Sung, and J.E. Yang. 2012. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 118:536-544.
Alvarenga, P., A.P. Goncalves, R.M. Fernandes, A. de Varennes, G. Vallini, E. Duarte, and A.C. Cunha-Queda. 2008. Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci. Tot. Environ. 406:43-56.
Alvarenga, P., A.P. Goncalves, R.M. Fernandes, A. de Varennes, G. Vallini, E. Duarte, and A.C. Cunha-Queda. 2009. Organic residues as immobilizing agents in aided phytostabilization: (I) Effects on soil chemical characteristics. Chemosphere. 74:1292-1300.
Beesley, L., and M. Marmiroli. 2011. The immobilization and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 159:474-480.
Borchard, N., K. Prost, T. Kautz, A. Moeller, and J. Siemens. 2012. Sorption of copper (II) and sulphate to different biochars before and after composting with farmyard manure. Europ. J. Soil Sci. 63:399-409
Brady, N.C., and R.R. Weil. 1999. The nature and properties of soils. 12th edition. Prentice Hall. Upper Saddle River, NJ.
Brewer, C.E., Y. Hu, K. Schmidt-Rohr, T.E. Loynachan, D.A. Laird, and R.C. Brown. 2012. Extent of pyrolysis impacts on fast pyrolysis biochar properties. J. Environ. Qual. 41:1115-1122.
Briggs, C., J.M., Breiner, and R.C. Graham. 2012. Physical and chemical properties of Pinus ponderosa charcoal: Implications for soil modification. Soil Sci. 177:263-268.
Cantrell, K.B., P.G. Hunt, M. Uchimiya, J.M. Novak, and K.S. Ro. 2012. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 107:419-428.
Cao, X., and W. Harris. 2010. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresource Technol. 101:5222-5228.
Chan, K.Y., and Z. Xu. 2009. Biochar: Nutrient properties and their enhancement. In: biochar for Environmental Management: Science and Technology. J. Lehmann and S. Joseph (eds.). Earthscan, London, UK. pp. 68-84
Ducey, T.F., J.A. Ippolito, K.B. Cantrell, J.M. Novak, and R.D. Lentz. 2013. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundance. Appl. Soil Ecol. 65:65-72.
Enders, A., and J. Lehmann. 2012. Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar. Commun. Soil Sci. Plant Anal. 43:1042-1052.
Espinoza, L., N. Slaton, and M. Mozaffari. 2006. Understanding the numbers on your soil test report. University of Arkansas Cooperative Extension FSA2118. Available at: http://www.uaex.edu/Other_Areas/publications/PDF/FSA-2118.pdf (verified 19 May 2015).
Fageria, N.K. 2001. Adequate and toxic levels of copper and manganese in upland rice, common bean, corn, soybean, and wheat grown on an Oxisol. Commun. Soil Sci. Plant Anal. 32:1659-1676.
Gaskin, J.W., R.A. Speir, K. Harris, K.C. Das, R.D. Lee, L.A. Morris, and D.S. Fisher. 2010. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 102:623-633.
Hamer, U., B. Marschner, S. Brodowski, and W. Amelung. 2004. Interactive priming of black carbon and glucose mineralization. Org. Chem. 35:823-830.
Hart, J., G. Pirelli, L. Cannon, and S. Fransen. 2009. Western Oregon and Western Washington pastures fertilizer guide. Available at: http://forages.oregonstate.edu/fi/topics/pasturesandgrazing/fertilizationandliming/pasturefertilizerguide (verified 19 May 2015).
Hart, J.M., D.M. Sullivan, J.R. Myers, and R.E. Peachey. 2010. Sweet corn (Western Oregon). Nutrient management guide EM9010-E. Oregon State University. Available at: http://ir.library.oregonstate.edu/xmlui/handle/1957/19064 (verified 19 May 2015).
Hass, A., J.M. Gonzalez, I.M. Lima, H.W. Godwin, J.J. Halvorson, and D.G. Boyer. 2012. Chicken manure biochar as liming and nutrient source for acid Appalachian soil. J. Environ. Qual. 41:1096-1106.
Ippolito, J.A. T.F. Ducey, K.B. Cantrell, J.M. Novak, and R.D. Lentz. 2014a. Designer biochar influences calcareous soil characteristics. In review with Chemosphere.
Ippolito, J.A., J.M. Novak, W.J. Busscher, M. Ahmedna, D. Rehrah, and D.W. Watts. 2012a. Switchgrass biochar effects two Aridisols. J. Environ. Qual. 41:1123-1130.
Ippolito, J.A., D.G. Strawn, K.G. Scheckel, J.M. Novak, M. Ahmedna, and M.A.S. Niandou. 2012b. Macroscopic and molecular investigations of copper sorption by a steam-activated biochar. J. Environ. Qual. 41:1150-1156.
Ippolito, J.A., M.E. Stromberger, R.D. Lentz, and R.S. Dungan. 2014b. Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Qual. 43:681-689.
Ippolito, J.A., M.E. Stromberger, R.D. Lentz, and R.S. Dungan. 2014c. The effect of hardwood biochar and manure addition on calcareous soil chemical and microbial characteristics. In review with Chemosphere.
Kammann, C.I., S. Linsel, J.W. Gobling, and H.W. Koyro. 2011. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil-plant relations. Plant Soil. 345:195-210.
Kloss, S., F. Zehetner, A. Dellantonio, R. Hamid, F. Ottner, V. Liedtke, M. Schwanninger, M.H. Gerzabek, and G. Soja. 2012. Characterization of slow pyrolysis biochars: Effects of feedstocks and pyrolysis temperature on biochar properties. J. Environ. Qual. 41:990-1000.
Knicker, H. 2007. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochem. 85:91-118.
Laird, D.A., P. Fleming, D.D. Davis, R. Horton, B. Wang, and D.L. Karlen. 2010. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma. 158:443-449.
Lehmann, J., J.P. da Silva Jr., C. Steiner, T. Nehls, W. Zech, and B. Glaser. 2003. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant Soil. 249:343-357.
Lentz, R.D., and J.A. Ippolito. 2012. Biochar and manure affects calcareous soil and corn silage nutrient concentrations and uptake. J. Environ. Qual. 41:1033-1043.
Lentz, R.D., J.A. Ippolito, and K.A. Spokas. 2014. Biochar and manure effects on net N mineralization and greenhouse gas emissions from calcareous soil under corn. Soil Sci. Soc. Am. J. 78:1641–1655.
Li, L., P.A. Quinlivan, and D.R.U. Knappe. 2002. Effects of activated carbon surface chemistry and pore size structure on the adsorption of organic contaminants from aqueous solution. Carbon. 40:2085-2100.
Moore, A., S. Hines, B. Brown, C. Falen, M. de Haro Marti, M. Chahine, R. Norell, J. Ippolito, S. Parkinson, and M. Satterwhite. 2014. Soil-plant nutrient interactions on manure-enriched calcareous soils. Agron. J. 106:73-80
Mulvaney, R.L., 1996. Nitrogen - inorganic forms. In: Sparks, D.L. (Ed.), Methods of soil analysis, part 3 - chemical methods. Soil Science Society of America, Madison, WI, pp. 1123-1184.
Namgay, T. B. Singh, and B.P. Singh. 2010. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Aust. J. Soil Res. 48:638- 647.
Nelson, D.W., and L.E. Sommers. 1996. Total carbon, organic carbon, and organic matter. In: Sparks, D.L. (Ed.), Methods of soil analysis, part 3 - chemical methods. Soil Science Society of America, Madison, WI, pp. 975-977
Novak, J.M., W.J. Busscher, D.L. Laird, M. Ahmedna, D.W. Watts, and M.A.S. Niandou. 2009a. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 174:105-112.
Novak, J.M., I. Lima, B. Xing, J.W. Gaskin, C. Steiner, K.C. Das, M. Ahmedna, D. Rehrah, D.W. Watts, W.J. Busscher, and H. Schomberg. 2009b. Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Ann. Environ. Sci. 3:195-206.
Parvage, M.M., B. Ulen, J. Eriksson, J. Strock, and H. Kirchmann. 2013. Phosphorus availability in soils amended with wheat residue char. Biol. Fertil. Soils. 49:245-250.
Reed, S.T., and D.C. Martens. 1996. Copper and zinc. In: Sparks, D.L. (ed.), Methods of soil analysis, part 3 - chemical methods. Soil Science Society of America, Madison, WI, pp. 703-722.
Rhoades, J.D., 1996. Electrical conductivity and total dissolved soilds. In: Sparks, D.L. (Ed.), Methods of soil analysis, part 3 - chemical methods. Soil Science Society of America, Madison, WI, pp. 417-435.
Soltanpour, P.N., G.W. Johnson, S.M. Workman, J.B. Jones, Jr., and R.O. Miller. 1996. Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In: D.L. Sparks (ed.). Methods of Soil Analysis, Part 3 - Chemical Methods. Soil Science Society of America. Madison, WI. pp. 91-139
Spokas, K.A, K.B. Cantrell, J.M. Novak, D.A. Archer, J.A. Ippolito, H.P. Collins, A.A. Boatang, I.M. Lima, M.C. Lamb, A.J. McAloon, R.D. Lentz, and K. Nichols. 2012. Biochar: A synthesis of its agronomic potential beyond carbon sequestration. J. Environ. Qual. 41:973-989.
Steel, R.G.D., and J.H. Torrie. 1980. Principles and procedures of statistics: A biometrical approach. 2nd Ed. McGraw-Hill, New York.
Thomas, G.W., 1996. Soil pH and soil acidity. In: Sparks, D.L. (ed.), Methods of soil analysis, part 3 - chemical methods. Soil Science Society of America, Madison, WI, pp. 475-490.
Uchimiya, M., K.B. Cantrell, P.G. Hunt, J.M. Novak, and S. Chang. 2012. Retention of heavy metals in a Typic Kandiudult amended with different manure-based biochars. J. Environ. Qual. 41:1138-1149.
University of California, Davis Soil Resource Laboratory. 2008. Soilweb: An online soil survey browser. Google Earth interface. Available at: http://casoilresource.lawr.ucdavis.edu/drupal/node/902 (verified 19 May 2015)
Van Zwieten, L., S. Kimber, S. Morris, K.Y. Chan, A. Downie, J. Rust, S. Joseph, and A. Cowie. 2010. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil. 327:235-246.
Ventura, M., G. Sorrenti, P. Panzacchi, E. George, and G. Tonon. 2013. Biochar reduces short-term nitrate leaching from A horizon in an apple orchard. J. Environ. Qual. 42:76-82.
Wardle, D.A., M.C. Nilsson, and O. Zackrisson. 2008. Fire-derived charcoal causes loss of forest humus. Science. 320:629-629.
Whiting, D., A Card, C. Wilson, and J. Reeder. 2011. Soil pH. Colorado State University Extension CMG GardenNotes #222. Available at: http://www.ext.colostate.edu/mg/Gardennotes/222.html (verified 19 May 2015).
Yuan, J.H., and R.K. Xu. 2011. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manage. 27:110-115.
APPENDIX
Biochar and Compost Characterization Methods
The biochar and compost total C and N were determined by a laboratory dry combustion method (Nelson and Sommers, 1996), whereby C and N are converted to CO2 and N2 gases and analyzed using an instrument called a Thermo-Finnigan FlashEA1112 CN analyzer (CE Elantech Inc., Lakewood, NJ). pH and electrical conductivity (or salt content) were determined on a saturated paste extract (Thomas, 1996; Rhoades, 1996), where each material is mixed with deionized H2O to make a mixture that glistens on the surface, flows only slightly when tipped, and the mixture slides cleanly off the mixing implement. NO3-N and NH4-N concentrations were determined using a 2M KCl extract (Mulvaney, 1996). NO3-N readily enters the extracting solution, while NH4-N is typically held more tightly onto biochar or compost particles. In order to remove NH4-N, scientists typically add excess of another positively charged ion such as K, in the form of KCl. The K replaces NH4-N on the material, the NH4-N enters the extracting solution, and then both NH4-N and NO3-N can be analyzed in the laboratory using chemical methods that develop specific colors for each constituent. The organic N content of biochar and compost was determined as difference between total N and inorganic N (i.e. NH4-N + NO3-N content). Total metal concentrations were determined using by a very strong acid digestion (i.e. perchloric-nitric-hydrofluoric-hydrochloric; Soltanpour et al., 1996). A small amount of material is placed inside a large glass test tube and the acids are added. Afterwards, the mixture is heated to help completely dissolve the material. The resulting solution is then analyzed on an instrument called an inductively coupled plasma optical emission spectrometer (ICP-OES). This instrument sprays the sample into very hot gas (up to 10,000 oC). This excites all of the elements present in the sample, with the elements giving off characteristic wavelengths of light specific for each element. The intensity of those wavelengths of light can be measured and then concentration of each element in the sample can be determined.
Soil Characterization Methods
All soils were collected within the top 30 cm of each plot. Soils were returned to the laboratory, air-dried, ground and passed through a 2-mm sieve and then analyzed for pH, EC, total C and N, NO3-N and NH4-N, and organic N as described above. Soil was also analyzed for inorganic C analysis and soil organic C was determined by difference between total and inorganic C. Additionally, soils were analyzed for Mehlich-III extractable (i.e., a measure of plant-available nutrients in acidic soils; the extraction technique is named after the scientist that invented procedure) Ca, Cu, Fe, K, Mg, Mn, Mo, P, and Zn (Reed and Martens, 1996). Elemental concentrations were determined using an inductively coupled plasma-optical emission spectrometer as previously described.
Vetch and Corn Characterization Methods
Vetch and corn samples were returned to the laboratory, dried in an over at 60 oC for 72 hours, and then ground to pass a very fine mesh screen. Total C and N concentration of the subsample was determined as described above. A small amount of plant sample was placed in a beaker and ashed in a furnace for 5 hours at 500oC. After cooling, a small amount of nitric acid was added and the samples were heated on a hot plate until all of the solid material was dissolved. Samples were then further diluted, passed through filter paper, and analyzed for total Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn using ICP-OES.


- Please write us your comment -
×