In the Pacific Northwest of the United States, where public support for solutions to climate change is strong, researchers and entrepreneurs are actively building a biochar industry. A robust biochar producing industry is highly desirable as current research and field use have begun to demonstrate biochar’s utility for increased soil water-holding capacity, fertilizer conservation, stormwater and roof drain filter media, contaminated site cleanup, animal feed supplement and carbon sequestration (Fuchs, et al., 2012). Researchers and public agencies are also investigating the environmental and economic implications for a variety of energy options as part of the biochar production process. The Washington State has supported a number of assessments and other programs to evaluate the potential of biochar to contribute to economic development, waste management, climate mitigation and healthy agricultural soils. These assessments are available as open source documents (see Sidebar).
The Washington Department of Ecology has funded an outreach and education program to engage the public in discussions about the beneficial uses of biochar. While the public is familiar with the idea of burning biomass for energy (wood home heat), there is a knowledge gap about biochar and the biomass thermochemical processes that produce biochar and energy products. One familiar example useful for explaining these processes to a general audience is a campfire.
Thermochemical Conversion around the Campfire
Biomass is made up of large carbon molecules of lignin, cellulose and hemi-cellulose, the so-called ligno-cellulosic compounds. The combustion (i.e. oxidation) of biomass such as wood in a campfire involves five easily distinguishable steps:
- Evaporation of the water and other small molecules with the formation of white smoke (from the release of water vapor as steam) at temperatures below 200°C.
- Biomass decomposition resulting in the release of an acidic, stinky, irritating smoke (indicating compounds like acetic acid in the vapors) at temperatures between 200 and 300°C.
- Biomass decomposition resulting in the release of brown or black smoke and formation of a flame at temperatures between 300 and 650°C. If enough air (oxygen) is present, the black smoke (gas phase oils and tars released from the heated biomass) will burn in the flame and very little smoke will be visible.
- The flame disappears and the remaining char glows orange and yellow as it oxidizes. Combustion flame (>650°C) can only be supported with volatile compounds (in the black smoke). Once those volatile compounds are gone, oxygen that reaches the biochar surface oxidizes the char carbon structure and releases heat. This oxidation occurs when the biochar surface reaches 400°C and can increase the char surface temperature to over 1000°C. This is the stage of combustion useful for grilling.
- A white or grey powder called ash is formed when all the carbonaceous material has been oxidized.
Drying:
The first step in our campfire experience is typically called “drying.” Feedstock (even bone dry) contains water. Kiln dry wood contains 12% to 20% water. Dry straw ranges from 10% to 15% moisture. Green biomass exceeds 40% moisture. Before energy or biochar can be produced from biomass, heat energy must be used to drive off moisture.
Torrefaction:
The second heating step is called “torrefaction” which happens between 200 300°C, typically in the absence of oxygen. Under these conditions, the breakdown of organic structures starts and the biomass becomes friable, or easily broken, reducing the energy required for grinding. This technology has found applications as a pre-treatment of biomass for coal burners (either alone or as part of co-firing strategies). Torrefaction followed by compressing the torrefied biomass into pellets is a strategy currently being studied to reduce transportation costs and to improve the handling durability of the fuel.
Pyrolysis:
Pyrolysis takes advantage of the phenomena described in steps 1, 2 and 3 of our campfire, and typically occurs between 350 and 600°C in the absence of oxygen. Biochar produced below 450°C is likely to still contain a large fraction of materials (lignin and tars from biomass decomposition) that can be further pyrolized. Sometimes these volatiles are released as vapors, only to re-condense on the biochar. The typical “campfire odor” found in some biochars is mainly due to the condensation of pyrolysis vapors on the char as the biochar is cooling. There are some auto-thermal (self-heating) kilns that produce biochar at higher temperatures, sometimes up to 750°C. The biochars produced at these higher temperatures tend to have higher surface areas and lower content of volatiles and oxygen. The surfaces of these biochars contain fewer oxygenated functional groups. Such groups are the chemical receptor sites where compounds can be adsorbed (adhere to) on the biochar. The rate at which the pyrolysis process occurs is important as well. Heating rates can be controlled to maximize either production of biochar plus volatile gases for direct burning (slow pyrolysis) or to maximize liquid energy products (fast pyrolysis).

Figure 1: Example of modern pyrolysis equipment: The Pyreg reactor one of the most successful commercial installations in Europe (Courtesy of Pyreg GmbH, http://www.pyreg.de/machinery-en.html).
Slow Pyrolysis:
In slow pyrolysis, the biomass is heated slowly and held at the highest treatment temperature for several minutes to days, depending on the feedstock, temperature and particle size. As the biomass is slowly charred, vapors are released that can be condensed and separated into a water-soluble fraction of acids and sugars, and an organic solvent-soluble fraction composed of phenol compounds. Figure 2 shows an example of a (very polluting) slow pyrolysis technology without heat or vapor recovery. Slow pyrolysis was used extensively by the old wood distillation industry (see Figure 3) to produce methanol (wood alcohol), acetic acid, and acetone, and is currently used in many developing countries as the main technology for charcoal production. Slow pyrolysis char may contain 25% to 35% of the original feedstock weight and up to 50% of the original carbon.

Figure 2: Environmental impact of slow pyrolysis carbonization units without recovery of volatile products (Courtesy of the Washington State Department of Ecology).
Campfires were the first step in the evolution of slow pyrolysis reactors. An important fraction of the char commercialized today in Africa is produced in earth kilns or earth pits (slow pyrolysis) using forest biomass or energy crops such as eucalyptus. An earth kiln is nothing more than a campfire covered with soil that acts as a barrier to protect the char from the attack of the oxygen which would reduce it to ash. For several centuries, the evolution of pyrolysis reactors was limited to changes in the shape of the ovens and the construction materials (brick, concrete, steel) used as barriers to oxygen attack. These small changes gave birth to a variety of kilns used today around the world, mostly for fuel charcoal production. These systems are often very polluting because the pyrolysis vapors are typically released to the atmosphere, creating risks to human health and the environment (figure 2).
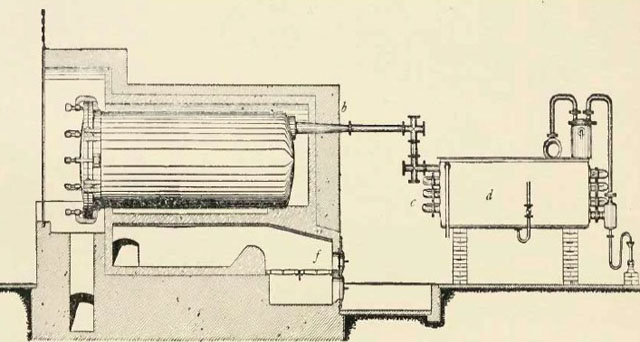
Figure 3: Horizontal steel retort with condenser for wood distillation (Klar, 1925).
A cleaner type of kiln that evolved from the campfire is the so-called open fire kiln. These simple systems, designed to restrict air (oxygen) access to the biochar, can be very clean, as the smoke is typically burned in the flame. Some examples of open fire kilns (and related technologies) are the Japanese cone kiln in Figure 5, the Kon Tiki Kiln (see Kon Tiki - The democritization of biochar production), and the top-lit open burn method used in forest debris management (Wilson, 2014).

Figure 4: A cone kiln based on the design from the Japanese Moki company (Courtesy of http://www.backyardbiochar.net).
Open air pits can also make biochar if carefully monitored and extinguished before the wood burns to ash. In fact, when our campfire is made in one of the metal rings common at many campgrounds and picnic areas, it can be used to make biochar. The ring excludes oxygen as the fire heats and pyrolyzes the wood within the ring. If you put the fire out with water while there are still glowing coals in the fire ring, you will make biochar.

Figure 5: A typical campfire ring found in American national and state parks (Courtesy of Steven P. Ng https://www.flickr.com/photos/62003279@N00/).
Fast Pyrolysis:
The objective of fast pyrolysis is to produce a liquid fuel and biochar is only a byproduct. Fast pyrolysis reactions occur in the same range of temperatures (350-600°C) as slow pyrolysis, but using finely ground feedstock that heats quickly, so vapors are released very rapidly. The vapors are quickly removed from the reactor (vapor residence time is often less than 2 seconds), with the use of an inert carrier gas to avoid secondary reactions, cooled and condensed into liquids. This process results in converting up to 75 % of the original biomass into bio-oil. Most biochar produced in this process is typically combusted to provide the heat energy needed to drive the fast pyrolysis reactions.
Gasification:
The partial oxidation of the products in steps 2 and 3 of our campfire at temperatures above 700-1400°C is the distinctive characteristic of biomass gasification processes. Gasification occurs under limited oxygen conditions such that the biomass is converted to “synthesis gas” (syngas) consisting of hydrogen, carbon monoxide, and methane. Syngas can be burned in a boiler for heat or an internal combustion engine to create shaft power using similar technologies as those for natural gas. Oxygen required by gasification is only 15 to 30% of the oxygen needed for complete combustion to ash. Gasification char typically contains 10% - 12% of the original biomass weight and only 25% - 30% of the original carbon. A recent gasification plant is shown in Figure 7.

Figure 6: Biomass gasification demonstration plant (Güssing, Austria).
Combustion:
Complete combustion includes the processes of pyrolysis and gasification, and results in high temperature (>1400°C) oxidation of biomass. The resulting flame oxidizes volatile gasses to carbon dioxide and water vapor while the biomass is turned to ash. Ash is the non-carbon compounds found in biomass, such as phosphorous and oxides of potassium, calcium, magnesium, sodium, iron and manganese. Ash may also contain low to moderate amounts of carbon depending on the extent of the combustion process. While the oxidation of the volatiles is a very fast process that occurs in the gas phase, the oxidation of the char is a relatively slow process that occurs when the oxygen attacks the biochar surface. Like a campfire, the amount of char produced can be controlled by quenching the glowing char (by dousing with water), spreading the embers out to the reduce heat, or by excluding oxygen from the char (covering the glowing char with soil or sealing it in an air-tight container).
Properties of Biochar
Biochar characteristics can vary widely depending on what the biochar was made from and how it was made. The most valuable characteristics of biochar are determined by how the biochar will be used. Typically, biochar characteristics and performance will depend on the content, composition and physical characteristics of the carbonaceous fraction (biochar) and the ash. The characteristics of biochar include its elemental composition (carbon, hydrogen, nitrogen, sulfur, and oxygen) and surface characteristics (surface area, pore size, surface chemistry). One way to characterize biochar that comes from solid fuel evaluations is proximate analysis, which measures the relative fractions of moisture, fixed carbon, volatiles and ash in biochar. The impact of biochar characteristics on soil fertility will depend on the nature of the soil where it is to be applied. Properties which are useful in many soil settings are: high (>70%) stable carbon content, low ash content, high surface area ( around 300 m2/g), low volatiles content (from re-condensation of smoke), moderate pH (7 - 9.5), and capacity to neutralize acidic soil. Biochars with higher contents of ash or volatiles can still also be valuable soil (or compost) amendments depending on soil conditions and plant needs.
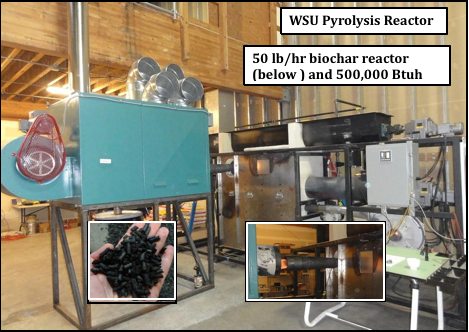
Figure 7: A research pyrolysis reactor located at Washington State University developed by Dr. Jerry Whitfield (Courtesy of Tom Miles).
Processing temperature is a key determinant of biochar properties. When thermal conversion temperature increases, the amount of volatiles remaining in the biochar decreases, while the stable fixed carbon and ash contents increase. Accordingly, as conversion temperature increases, so does the long term stability of the resulting biochar. The surface area of biochars produced at lower temperatures (< 500°C) are in general below 150 m2/g depending also on the feedstock. An upper temperature boundary, typically between 700 and 800°C exists at which the surface area of biochars begins to decrease (Downie, et al., 2009).
Naturally occurring oxygenated compounds on the surfaces of biochar gradually decrease as the pyrolysis temperature increases. These compounds create slight negative charges, giving biochar the capacity to adsorb cations (positively charged ions) common in many fertilizers. Wood biochars produced above 600°C have had most of the oxygen-containing compounds driven off, which gives them a neutral to positively charged surface. This results in lower cation exchange capacities (i.e. the ability to hold and slowly release fertilizer cations such as calcium, magnesium, potassium and ammonium).
Independent of the temperature, herbaceous feedstock (grass, straw, etc.) chars typically have higher ash contents than woody feedstock chars. This is not surprising in that woody biomass is the result of perennial biomass fixing atmospheric carbon into long-chain carbon compounds (cellulose) and lignin (nature’s glue), which account for wood’s strength. In contrast, herbaceous feedstocks are the result of annual growth high in minerals (calcium, potash, phosphorous, etc.), which yield ash. Ash content drives up pH and creates acid neutralizing potential. Ash will also bring with it calcium, magnesium, phosphorous, potassium and iron, all fertilizer components. Ash can be highly caustic (dangerous waste in Washington is designated at pH >11.5) and too much ash may be detrimental to plant growth.
All of these biochar characteristics can be analyzed in advanced laboratories. However, some biochar characteristics can also be easily determined by inspecting the product with our senses.
Hands-on Assessment of Biochar
Char and ash are familiar materials to anyone who has made a campfire. Generally, it’s assumed that char is pure carbon and always black, while ash is a light color and fluffy; this is not always the case. Ash can appear black, but still be mostly ash. Char can be mostly pure carbon or it can include a large fraction of volatiles/tar containing oxygen and hydrogen. A simple way to determine the presence of volatiles in chars, which can be harmful to soils, is to smell the char. Does it smell like a campfire pit (creosote) or does it have little noticeable odor?
Another way to evaluate char quality is to touch it. Is the char oily? Does it leave a smear on your fingers requiring more than water to rinse away? Oily smears indicate organic compounds that have re-condensed on the char. These can include polyaromatic hydrocarbons (PAH) compounds that may present health hazards. Oily char suggests caution. These chars may be acceptable to go into other processes such as compost where the microbiology will metabolize the non-stable carbon components. Direct soil application of oily char may support bacteria growth that reduces nitrogen availability and thus competes with plant growth.
An interesting method to compare the ash content between biochars is to observe the level of effervescence when a small amount of the char is put in contact with several drops of vinegar (5% acetic acid) or muriatic (hydrochloric) acid. Muriatic acid is stronger than acetic acid and will yield greater effervescent response for a given char. Chars containing high ash will create great fizz even with off the shelf vinegar. Apply several drops of the vinegar onto the char and watch and listen for effervescence.
Biochar can vary in density (density is the weight per volume) depending on the process, remaining non-charred volatiles (un-reacted biomass) content, ash content, and pyrolysis quench method. Combustion may be stopped by quenching with water or other means of cutting off oxygen. A dry char, in some cases, can be as “light” as 72 kg/m3 (4.5 lbs/ft3). A char density greater than 180-190 kg/m3 (10-12 lbs/ft3) indicates a higher moisture content, a higher content of volatiles or ash, or a combination of these. Because of the potential variation in density, biochar should be measured by volume for application purposes. To approximate dry mass, chars can be air-dried like soil samples, weighed, then oven dried at 105°C for 24 hours, and weighed again.
A shake test can be another useful visual assessment of biochar characteristics. To conduct the test, add a volume of biochar into an equal volume of water in a sealed glass jar and shake. Heavy, free ash, gray in color, will fall to the bottom where it tends to pack like mineral sand. The ash in this layer tends to resist moving when jarred, a characteristic that helps distinguish it from the less dense, blacker char sediment. While char absorbs water, some pore space remains filled with air and buoyant, a condition that allows the fine char to slosh or move relative to the ash when the glass container is jarred. Measure the depth of the ash relative to the total thickness of biochar in the jar. This is the proportion of ash. The shake test ash proportion will be lower than lab determined ash for two reasons. First, the lab determinations are made on a weight basis, but the shake test observations are necessarily on a volume basis. Second, the lab determines total ash, which includes any ash constituents held within the biochar bulk matrix, whereas the shake test only shows the smaller proportion of free ash.
The shake test can be extended to assess biochar water absorption. With air trapped in pores, the coarsest char component will initially float. The proportion of unabsorbed water remaining below the float and above the sediment provides a gauge of the absorptive capacity of the biochar. To assure repeatable results, wait to observe until most of the biochar has sunk below the water line, and the floating layer has thinned. Decant free water for a more refined measurement.
These simple tools can be used to guide an intuitive approach to char properties and therefore, quality. However, the authors believe that biochar available for sale at quantity should be thoroughly characterized by a qualified laboratory.
Putting it All Together for Public Education
Taking biochar education to the public is a crucial step in not only capturing the myriad environmental and social benefits of biochar, but in building markets that support small-scale local industry. Complexities in biochar production processes and biochar characteristics can make biochar seem to be the province of scientists only. However, in spite of these complexities, biochar is completely accessible; given proper instruction, anyone with biomass and a match can create biochar. With more knowledge and materials, people can create a cottage industry around biochar markets that develops appropriate technology, improves on existing technologies, treats biomass waste and sells biochar.
The current outreach method in educating the public in the state of Washington focuses on grass-roots community organizing. An innovative approach, using State Public Participation grant funding, has been to take biochar education into rural communities in counties with significant excess biomass. This program, Biochar Community Conversations (BCC), relies on bringing expert knowledge and demonstration directly to communities. Through direct conversation, biochar’s properties and production are demystified; all of biochar’s benefits, including biomass waste reduction, are examined in the specific local community context.
Outreach begins with county and tribal leaders in government, public utility districts, natural resource/restoration agencies and organizations, agricultural extension, conservation districts, economic development, education, conservation organizations and biomass-related industries. These people have unique knowledge of local conditions and a list of problems they need to solve. The BCC process starts by introducing these leaders to biochar and then listening to understand and address county-specific problems where biochar can be part of the solution. Problems identified through outreach become the focus of that county’s BCC.

Figure 8. Phil Small demonstrates the open fire method of making biochar to interested citizens in Cedonia, Washington (Courtesy of Gloria Flora).
Three to four key communities are selected in each county based on geographic distribution, community issues, and local resources. No town is too small to be considered. Some of the greatest participation, per capita, has occurred in towns with populations of 100-200 people. Additionally, public education programs typically require those residents to travel some distance to a large town and tend not to be tailored to their communities’ specific needs. Each community is visited twice, about two to four weeks apart. The first evening presentation focuses on introducing biochar, its benefits and potential feedstocks. The second evening presentation dives more deeply into the characterization of biochar and production techniques/technologies. Technology focuses on affordable, do-it-yourself opportunities as the first step. By actually making biochar, people will more readily understand and be able to use it. Scheduling two presentations allows for more information to be shared and having time between the presentations allows for deeper, richer questions because people have had time to contemplate local applications. The second presentation always has more attendees than the first as word spreads and people bring others with them. Although the time set for formal presentation and discussion is only 1.5 hours, BCCs often run far longer as people discuss community options and opportunities together.
Raising awareness of biochar and its benefits through the BCCs has lead to numerous presentations in additional venues. But with BCCs themselves, the benefits of bringing people together in a problem-solving setting, arming them with unique potential solutions and providing expertise to answer tough questions cannot be understated. Initial feedback has been very positive. Although attendance has been lower than desired, the interest level and continued engagement has exceeded expectations. As the program continues, monitoring is key; results will be shared at the program’s conclusion and will include advice on presentation content, delivery techniques, hands-on content, effective advertising, ancillary demonstrations, and future BCC-themed events and programs.
The biochar reports and the public outreach programs developed in the state of Washington are examples of useful tools for building a biochar industry globally that can go some distance to counteract the terrible effects of climate change. The authors welcome your comments and feedback on our work.


Community Gardens and Local Fire Bylaws
Do you have a strategy for convincing local fire departments to allow on site biochar making by community gardeners? Specifically, the local council has arranged for delivery of chipped plant waste to be available for making compost but making biochar on site would be prohibited with local rules.